Our history
The 1990s
Epitech Group’s research activity begins: following in the footsteps of Rita Levi Montalcini, a group of young researchers understand the importance of a pharmacological intervention to modulate the so-called “non-neuronal” cells (mast cells, microglia and astrocytes), involved in the control of peripheral and central nervous sensitivity, to counteract neuroinflammation and pain common to various pathologies.
Studying the biological role of these cells led the brilliant research group to identify, in collaboration with Prof. Montalcini’s laboratories, the mechanism that regulates the reactivity of non-neuronal cells, which they define as “ALIA” (Autacoid Local Injury Antagonism), thanks to the endogenous substance Palmitoylethanolamide (PEA).
1990s – Brainstorming between Francesco della Valle, Founder of Epitech Group, and Rita Levi Montalcini. © Epitech Group SpA
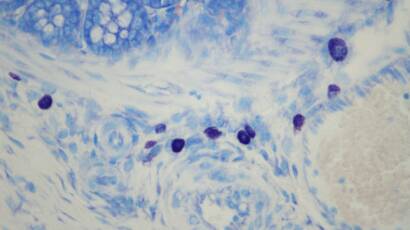
Mast cells (purple) in colon submucosa.
Courtesy of Dr. Elena Lucarini, Postdoctoral Researcher, University of Florence (NEUROFARBA). © Epitech Group SpA
1994
A patent application is filed for the protection of the method for synthesising exogenous Palmitoylethanolamide (F. della Valle et al.).
2002
An application is filed for the first patent protecting a body-usable form (micronised) of exogenous palmitoylethanolamide (F. della Valle et al.).
Our research shows us that the palmitoylethanolamide obtained using the previously-patented method of synthesis has poor bioavailability when administered systemically. We realise that particle size is a critical issue.
2004
As a result of our research, the company enters the human health market with the application of the ALIA mechanism to the podological dermatology setting.
2006
The innovative micronization technology, which makes PEA bioavailable, allows us to develop modulation strategies without side effects and particularly effective on chronic-neuropathic pain and neurodegenerative diseases.
The company launches Normast 300 mg, the first product containing a bioavailable form of Palmitoylethanolamide, supported by pre-clinical and clinical evidence.
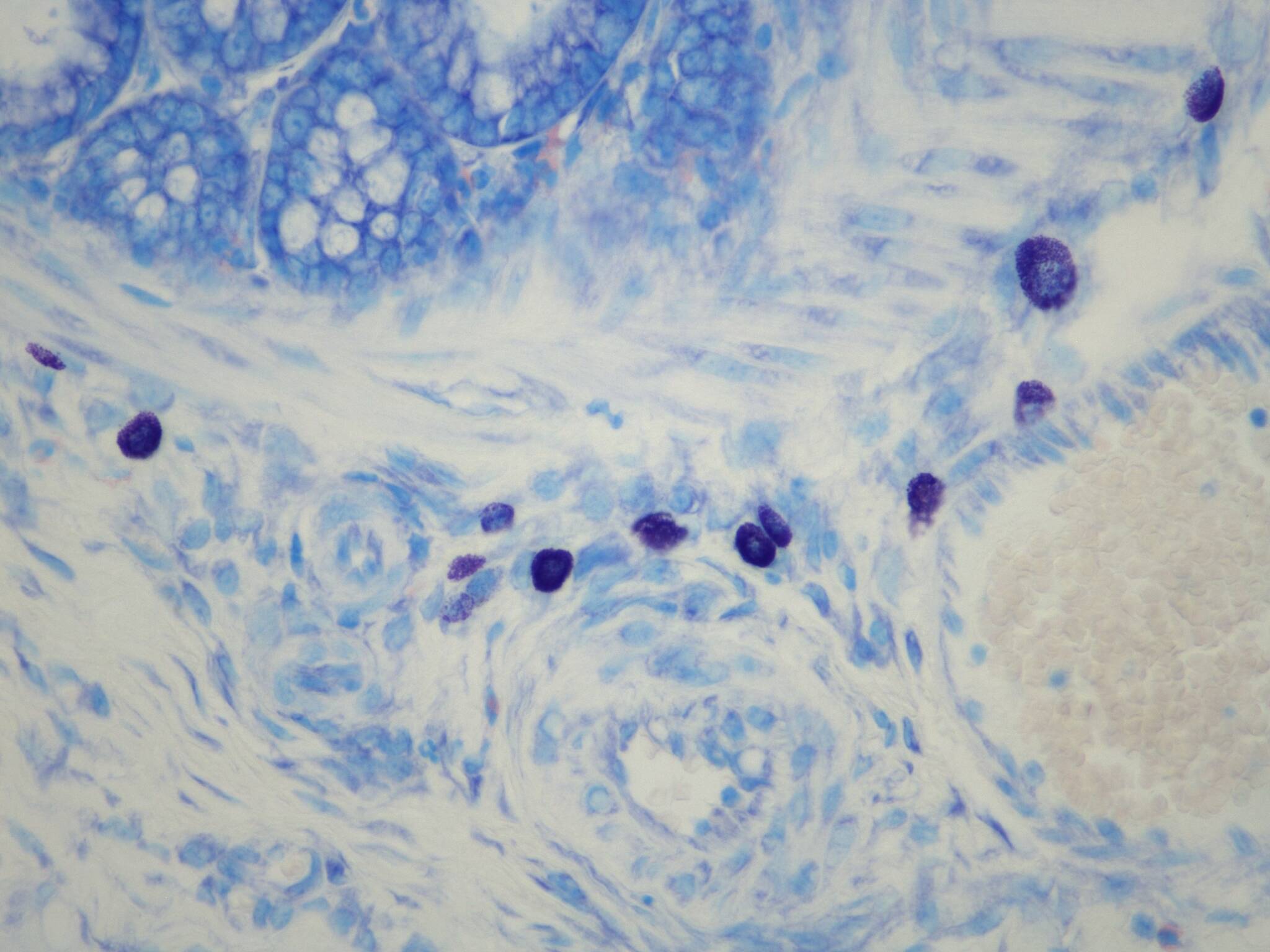
Mast cells (purple) in colon submucosa.
Courtesy of Dr. Elena Lucarini, Postdoctoral Researcher, University of Florence (NEUROFARBA). © Epitech Group SpA
2008
Epitech Group starts attracting the attention of the scientific and business worlds at international level. We sign the first licensing agreements for molecules developed by Epitech Group.
2015
Our commitment to research continues to grow with the opening of two local preclinical units in Messina and Pozzuoli.
Epitech Group becomes a joint-stock company and obtains Quality System certification (ISO 9001: ISO 13485).
2017
The company’s inclusion in the list of innovative small and medium-sized enterprises is a formal acknowledgement of our spirit of innovation.
2019
Thanks to its fervid research activities, Epitech Group expands its baggage of innovation: in 2019 our patent portfolio consists of 262 international patents covering the therapeutic use of ALIAmides.


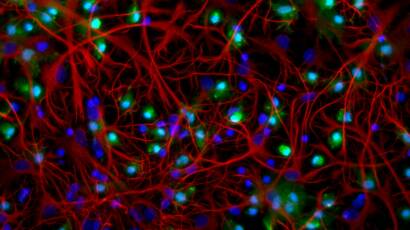
Co-coltura di microglia e astroglia. © Epitech Group SpA
2020
We open a new facility, a new technical department with chemistry laboratories and equipment for producing topical products.
2023
We move to a new company headquarters in order to accommodate the company’s growing workforce. Between 2018 and 2023, Epitech Group doubles its turnover.
2025
In the first quarter we crossed the threshold of 400 international patents granted on molecules conceived by our researchers. A result that materializes the constant increase in knowledge on neuroinflammatory mechanisms, and that paves the way to important future developments.
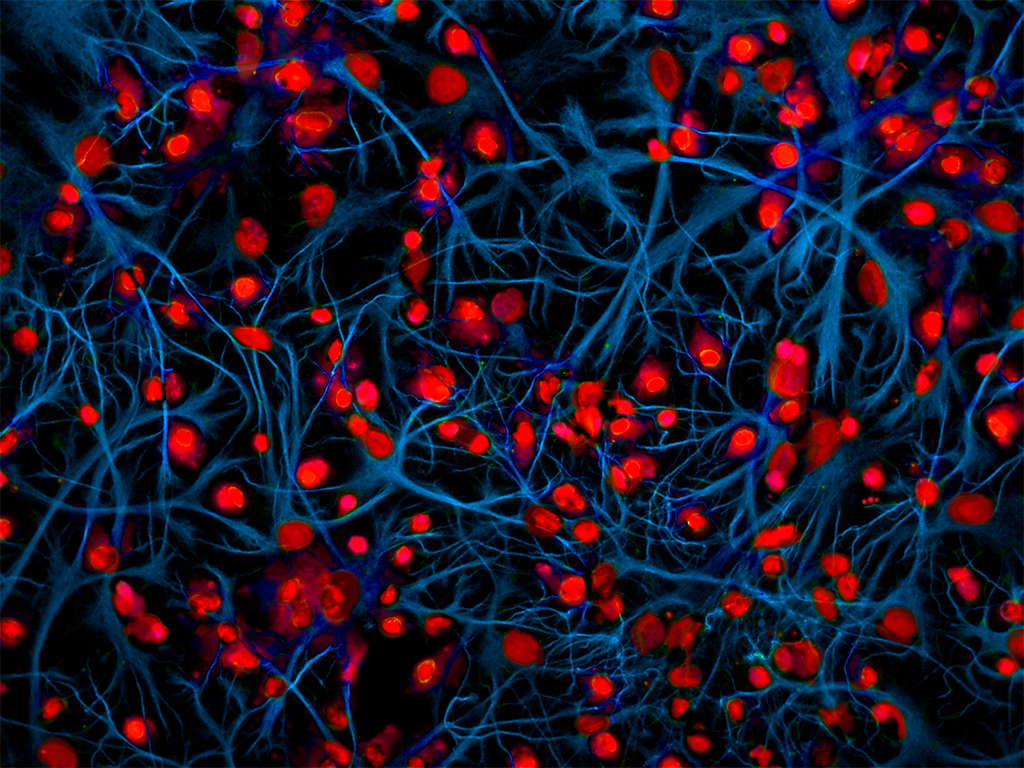
Microglia and astroglia co-colture. © Epitech Group SpA


